Recall & Field Corrective Action Services
All-In-One Recall & Field Corrective Action Services
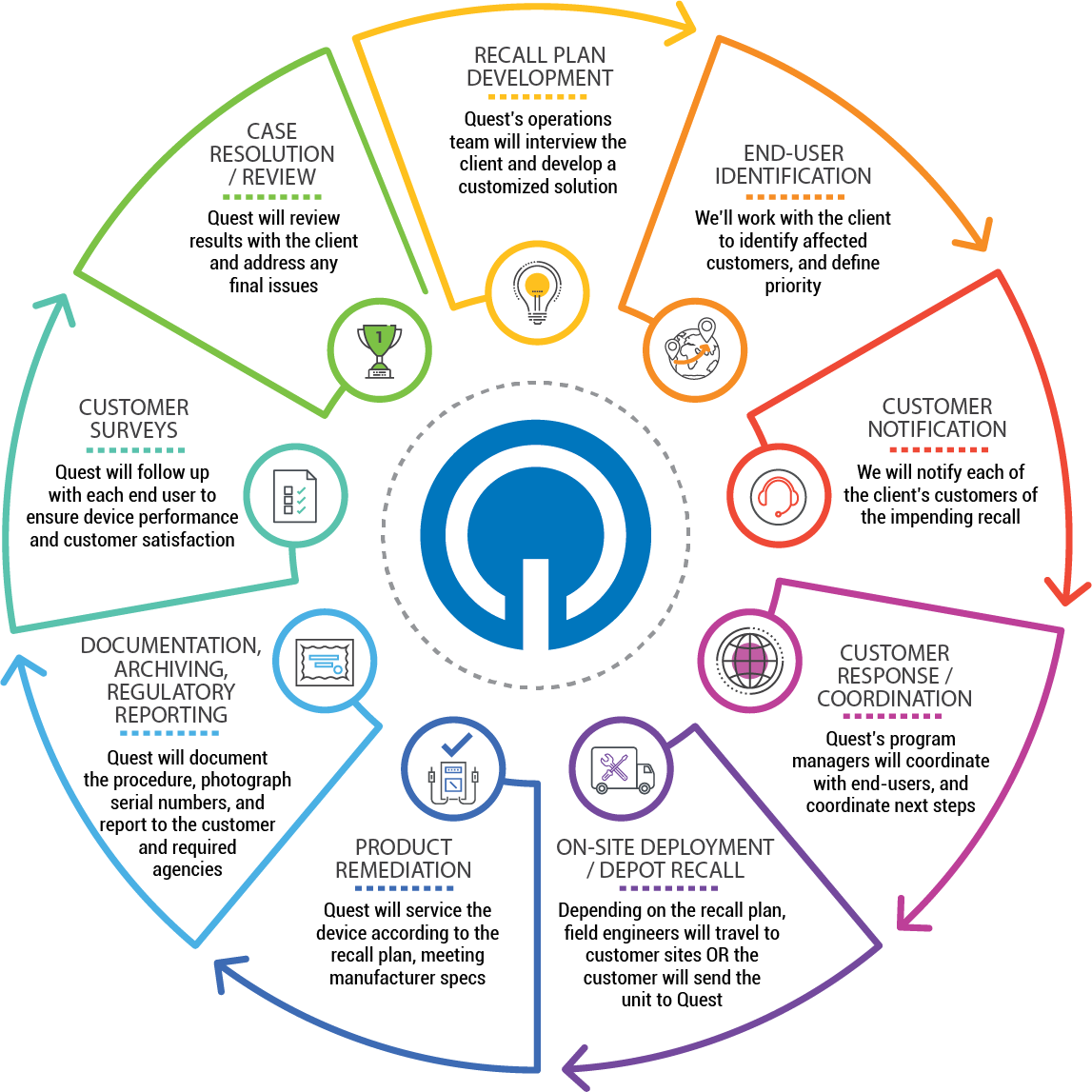
Quest brings a predictable cost model to unpredictable events
Click Image to Enlarge
Medical devices are engineered and manufactured with the highest level of precision and quality to ensure they perform reliably — but nothing is full-proof. Only in a real-world setting can devices be put to the test, demonstrating their ability to withstand unpredictable and sometimes extreme conditions. If a design flaw is discovered in the field, it is critical that the medical device manufacturer swiftly reacts and remediates the situation to prevent the possibility of causing a patient harm. No device manufacturer ever plans a corrective action, rework or recall, nor is it within the core competencies of the quality and regulatory teams to assemble and execute programs of this magnitude without disrupting routine business functions. Product corrective actions and recalls impact hundreds of device manufacturers every year, and how these actions are handled could make or break its reputation within the healthcare industry.
Whether your healthcare organization needs to perform these actions in the field by traveling directly to a customer site or rapidly expand repair facilities to accommodate units, Quest brings a fit-for-purpose approach to Recall Management and Field Corrective Action (FCA) services with a predictable cost model. Quest’s All-In-One Program is customized to fit your specific needs. It is developed using various components from its Call Center, Depot, Field, and Logistics solutions, which will handle the end-to-end process from outreach to remediation and all documentation. Quest can devise a flexible, successful recall program on your behalf, while increasing overall customer satisfaction.
The Quest Advantage
